Pfizer Covid Vaccine for Children Under 5 May Face Retraction
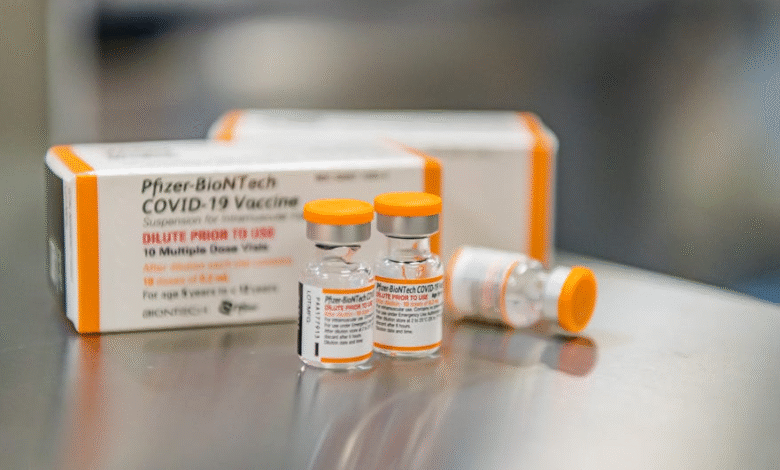
The Pfizer Covid vaccine for children has been a crucial part of the ongoing efforts to protect younger populations from the virus. However, recent developments indicate that the FDA may be considering retracting its authorization for this vaccine for kids under 5 years old. This potential move raises numerous questions about available protection for young children, especially as U.S. health agencies have shifted their stance on immunization policies under the new leadership of Health and Human Services Secretary Robert F. Kennedy Jr. Meanwhile, the CDC Covid vaccine guidance has evolved, suggesting that healthy children might still receive the vaccine if deemed necessary by healthcare providers. As Moderna continues to roll out its own vaccine for children, the landscape of pediatric vaccinations is rapidly changing, leaving families seeking clarity on the best course of action.
Discussing the pediatric Covid vaccine landscape reveals significant uncertainties, particularly surrounding the Pfizer Covid vaccine for young children. Recent announcements from the FDA concerning possible retraction of the vaccine’s authorization for children under 5 years have raised alarms among parents and healthcare professionals alike. While Covid often presents mild symptoms in children, those with specific health vulnerabilities may face heightened risks. In this context, updated guidance from the CDC allows for vaccinations based on a doctor’s assessment. As the conversation evolves, parents are turning their attention to the alternatives available, including the Moderna vaccine for children, while navigating these shifting recommendations.
Ongoing FDA Review of Pfizer Covid Vaccine for Children
The Food and Drug Administration (FDA) is currently re-evaluating the authorization of the Pfizer Covid vaccine for children under the age of 5. This decision has stirred concerns among parents and healthcare providers, especially as the health department considers retracting the emergency use authorization that has been in place for this age group. The FDA’s move comes amid a climate of heightened scrutiny regarding pediatric vaccinations, largely influenced by recent public health narratives, especially with the views espoused by vaccine skeptics, leading to confusion regarding Covid vaccine authorization under 5.
Pfizer has expressed its intent to continue discussions with the FDA to maintain the authorization for the upcoming fall and winter seasons despite the current reconsideration. They emphasize that the deliberations do not reflect concerns about the vaccine’s safety and efficacy but rather ongoing regulatory evaluations. It is crucial for parents to stay informed about these developments, as the absence of available vaccinations could significantly impact the protection of vulnerable children who are at an increased risk of severe Covid outcomes.
Comparative Analysis of Covid Vaccines for Children
While the FDA’s potential retraction specifically targets the Pfizer Covid vaccine for children under 5, it is essential to compare this with Moderna’s vaccine, which recently gained full approval for children with certain health conditions. This approval underscores the evolving landscape of Covid vaccination guidelines and highlights the CDC’s role in shaping the vaccination framework. The challenges faced by Pfizer may prompt parents to explore alternative vaccines, which includes the Moderna vaccine for children, particularly for those who may have existing health vulnerabilities.
Both the Pfizer and Moderna vaccines utilize messenger RNA (mRNA) technology, a revolutionary approach that instructs cells to produce a harmless piece of the virus to prompt an immune response. However, the ongoing discussions around the FDA’s stance on Pfizer’s vaccine could lead to a reevaluation of how other vaccines are perceived in the public domain. Increased scrutiny from authorities like the CDC has created an environment where parents are more discerning about Covid vaccine guidance and the overall safety for children.
Implications of CDC Guidelines on Covid Vaccination for Kids under 5 years Old and Modifications Post FDA Retraction Considerations
In light of the FDA’s considerations regarding Pfizer’s Covid vaccine, the CDC has been adjusting its recommendations for administering vaccines to healthy children under 5. Following earlier recommendations that included healthy children in the vaccination strategy, the removal of this recommendation has significantly changed the landscape of pediatric vaccination. The CDC’s nuanced stance now indicates that Covid vaccines may be administered based on a doctor’s assessment rather than as a blanket policy, reflecting a shift in how authorities are addressing childhood Covid vaccinations.
This pivot raises critical questions for parents as they navigate vaccination decisions. The option for doctors to decide based on individual health assessments emphasizes personalized healthcare, but it also requires parents to engage more proactively in discussions regarding vaccination. The ongoing modifications by the CDC underscore a broader dialogue about risk management and the importance of maintaining a robust vaccination strategy amid public uncertainty surrounding Covid vaccines for younger populations.
The Role of Health Agencies in Shaping Covid Vaccine Policy for Children
Health agencies like the FDA and CDC play a pivotal role in shaping vaccine policy and public health guidelines. Their ongoing evaluations of products like the Pfizer Covid vaccine are crucial not just for safety and efficacy but also for public trust in vaccination programs. As Secretary Robert F. Kennedy Jr. has emerged as a controversial figure critiquing vaccine authorizations, health agencies are now under immense pressure to justify their recommendations through transparent data and sound scientific reasoning.
This added scrutiny can lead to a paradox where potential beneficial vaccines might be sidelined, jeopardizing public health. The dynamic between maintaining a favorable profile for vaccines and addressing public concerns must be managed with diligence. Additionally, with the emphasis on the Moderna vaccine for children receiving renewed approval, agencies must communicate reassuringly about the comparative risks of not vaccinating based on their assessments of Covid vaccine safety, especially for children with specific health concerns.
Public Perception and Misinformation Regarding Pediatric Vaccination
Amidst the continuing debate around the safety and efficacy of the Pfizer Covid vaccine for children, public perception is critically affected by misinformation. The discourse surrounding the FDA’s potential retraction has opened the floodgates for uncertainties, compelling parents to question the validity of vaccines recommended by health authorities. The role of vaccine skeptics and their influence can strongly skew public opinion, making education and clear communication from health organizations even more vital.
Efforts must be put forth to counter misinformation and bolster public confidence in vaccines through data-driven messaging. Organizations should focus on highlighting the established benefits of vaccination, addressing common concerns raised by parents, and stressing the comparative safety of pediatric vaccines. Effective outreach can help dispel myths and reaffirm the importance of immunizing against Covid in vulnerable populations, ensuring that children under 5 have the protection they need to thrive.
Impact of Political Leadership on Vaccine Authorization
The shift in vaccine authorization views correlates with the political climate, particularly with the recent appointment of Secretary Robert F. Kennedy Jr. His influence has led to a reevaluation of existing vaccine policies, spawning apprehensions regarding the implications of political leadership on public health strategies. Moving forward, the balancing act between political perspectives and scientific integrity will determine how vaccines like the Pfizer Covid vaccine for children are authorized and perceived.
Political leadership can greatly affect public trust in health agencies. As critiques of vaccine policies increase, transparency and a commitment to scientific advisement become paramount for maintaining that trust. The resulting unease over vaccination protocols might challenge healthcare systems as they adapt to the evolving landscape of public perception and regulatory practices surrounding Covid vaccinations.
Navigating Parental Concerns About New Health Policies
Parents are facing an increasingly complex decision-making landscape regarding vaccinations for their children. The possibility of the FDA retracting the authorization for the Pfizer Covid vaccine introduces uncertainty that parents must navigate thoughtfully. Open lines of communication with healthcare providers become essential, as parents need accurate, up-to-date information to make informed choices about their children’s health.
Support from pediatricians and clear health guidelines play crucial roles in alleviating parental fears during this ambiguous time. By fostering trust and understanding, healthcare professionals can help parents weigh the risks associated with Covid in unhealthy children against the benefits of vaccination. Clear recommendations from agencies such as the CDC can help parents feel more informed and empowered in their decisions regarding Covid vaccine authorization under 5.
Future of Covid Vaccination Strategies for Young Children
The future of Covid vaccination strategies for young children hinges on how regulators respond to ongoing evaluations of vaccines like Pfizer’s. Should the FDA decide to retract emergency authorization, healthcare strategies will need to shift. Alternatives must be explored to safeguard children under 5 from the virus, ensuring that options are available for those who might be at higher risk due to underlying health concerns or exposure risk.
Innovation in vaccine development will also play a part in shaping future strategies, encompassing potential modifications or new products that could meet safety guidelines. Maintaining flexibility and adaptability within health policy frameworks concerning pediatric vaccinations will be key to responding effectively to any future health crises.
The Importance of Ongoing Research in Pediatric Vaccination
Ongoing research into the safety and efficacy of Covid vaccines for children is vital as health authorities navigate the complexities of vaccine authorization. The FDA’s decision-making process will benefit greatly from robust data highlighting the impact of vaccines on younger populations. Continuous monitoring of vaccine outcomes will not only reinforce public trust but also guide future vaccine policies based on evidence rather than public sentiment.
Moreover, collaboration between vaccine manufacturers, regulatory bodies, and healthcare professionals is essential for advancing the immunization framework for children. Fostering a culture of research and data sharing can enhance innovation, ultimately leading to improved health outcomes and solidifying vaccine strategies that prioritize children’s health during emerging infectious disease challenges.
Frequently Asked Questions
What is the current status of the Pfizer Covid vaccine for children under 5?
As of now, the FDA is considering retracting the emergency use authorization of the Pfizer Covid vaccine for healthy children under the age of 5. Discussions are ongoing between Pfizer and the FDA regarding potential paths forward to keep the authorization active for the upcoming winter season.
Is the Pfizer Covid vaccine safe for children under 5?
The Pfizer Covid vaccine continues to demonstrate a favorable safety profile according to the company. Although the FDA’s deliberations are occurring, they are not related to the vaccine’s safety and efficacy, ensuring that it remains an option for certain children at higher risk.
What should parents know about the CDC’s guidance on the Covid vaccine for children?
The CDC’s updated guidance indicates that Covid vaccines, including the Pfizer vaccine for kids, may be administered to healthy children if deemed necessary by a healthcare provider. However, the agency has removed its broad recommendation for healthy children and pregnant women, reflecting a shift in vaccination policy.
How does Pfizer’s Covid vaccine for children compare with Moderna’s vaccine?
Both Pfizer and Moderna vaccines for children utilize messenger RNA technology. However, Moderna’s vaccine has received full FDA approval specifically for children with health conditions that put them at higher risk for severe Covid illness, while Pfizer’s authorization for healthy children under 5 is under reconsideration by the FDA.
What are the implications of the FDA retracting Pfizer’s vaccine authorization for under-5s?
If the FDA retracts its authorization for the Pfizer Covid vaccine for children under 5, it could limit vaccination options for many young children, particularly those with underlying health conditions who may be at higher risk for severe outcomes from Covid.
What do parents need to know about Covid vaccine options for children?
Parents should be aware that there are options available for Covid vaccination, including the Pfizer Covid vaccine for children and Moderna’s vaccine. The availability of these vaccines may depend on FDA authorizations and the specific health status of their children.
Are there special considerations for children under 1 year regarding the Covid vaccine?
Yes, infants under 1 year of age are considered at higher risk for severe illness from Covid. This group may benefit from vaccination if approved options are available, highlighting the importance of consulting with a healthcare provider regarding the Pfizer Covid vaccine for children.
What recent changes have occurred regarding Covid vaccine recommendations for children?
Recent changes include the CDC’s revised guidance, which no longer broadly recommends Covid vaccinations for healthy children and pregnant women. However, vaccines may be considered on a case-by-case basis by doctors, particularly for those children at higher risk.
| Key Point | Details |
|---|---|
| FDA Review | The FDA is considering revoking the emergency use authorization of Pfizer’s Covid-19 vaccine for children under 5. |
| Implications | This could leave many healthy children without vaccination against Covid-19. |
| Ongoing Discussions | Pfizer is in discussions with the FDA about keeping the authorization active for the fall and winter. |
| Vaccine Safety | The FDA’s decision is not related to vaccine safety or efficacy, which remains favorable according to Pfizer. |
| Moderna’s Role | Moderna’s vaccine is fully approved but only for children with specific health conditions. |
| CDC Recommendations | The CDC has updated its guidance, allowing Covid vaccines for healthy children based on a doctor’s discretion. |
Summary
The discussion surrounding the Pfizer Covid vaccine for children has taken a new turn, as the FDA considers retracting its authorization for children under 5 years of age. This potential decision raises significant concerns regarding vaccination access for young children amid the pandemic. Despite the favorable safety profile of the vaccine, the evolving landscape of public health recommendations and vaccine availability necessitates careful consideration by parents and caregivers. It is crucial to stay informed on the latest guidance from health agencies as the situation develops.